Products and Pipeline
Products
Our goal is to develop small-molecule and protein best-in-class therapies that may significantly extend and improve the quality of life for those living with muscle diseases. We always put people first. That’s why we’re targeting areas of high unmet medical need with a sense of urgency while remaining open-minded and flexible so we can find new treatments as quickly as possible.
Progress
Sarcomatrix is committed to developing Laminin-111 as a potential treatment for LAMA2-RD and is seeking funding to complete the pre-clinical work and advance the program to human studies as soon as reasonably possible.
Our small molecule α7β1 integrin activators have shown promise in mouse models stimulating muscle regeneration and may support many forms of muscular dystrophy including Duchenne, Beckers, Emery-Dreifuss, Facioscapulohumeral, Limb-Girdle and many forms of Congenital muscular dystrophy.

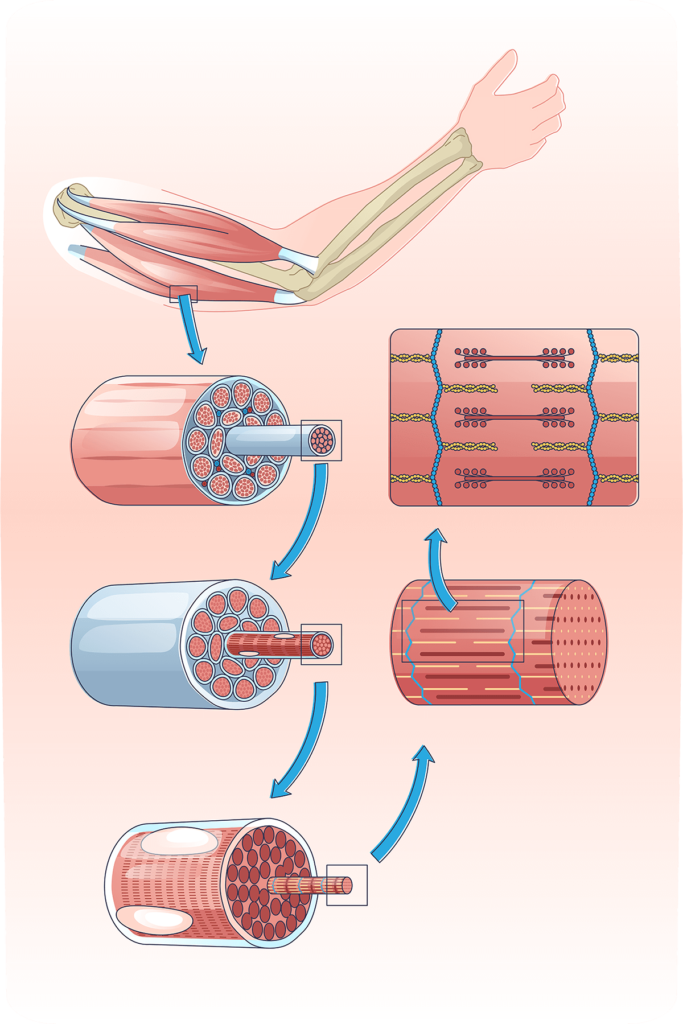
α7β1 Integrin Activators
Protein Replacement

We Believe Polytherapy is Key
The future of gene and cell therapy for muscular dystrophy is exciting, and further advancements in these fields hold promise for the development of effective treatments for these debilitating diseases. Sarcomatrix fully supports development of gene and cell therapies and looks forward to the day the cure afflicted individuals. Until that day comes, we’re committed to polytherapy.
Polytherapy involves using multiple treatments in combination to achieve the best possible outcome. With the current options, a single treatment may not be enough to address all the symptoms and underlying causes of the disease.
What sets us apart is our promising platform of small molecules and proteins which may be used alone or in combination with exon skipping, gene editing or gene therapy technologies.
Our Pipeline
Sarcomatrix’s research pipeline consists of α7β1 integrin activators and protein replacement therapies like Laminin-111. Our medicines are developed by doctors from the University of Nevada School of Medicine and Pharmacology in collaboration with the University of Illinois and the University of Minnesota. Although millions have been invested in our three lead therapies, more work is needed to complete pre-clinical research and move on to in-human trials.
S-969

Duchenne Muscular Dystrophy
Becker Muscular Dystrophy
Limb Girdle Muscular Dystrophy
Sunitinib

Duchenne Muscular Dystrophy
Becker Muscular Dystrophy
MD Cardiac Myopathy
LAM 111
Congenital Muscular Dystrophy
S-969

Duchenne Muscular Dystrophy
Becker Muscular Dystrophy
Limb Girdle Muscular Dystrophy
Sunitinib

Duchenne Muscular Dystrophy
Becker Muscular Dystrophy
MD Cardiac Myopathy
LAM 111
Congenital Muscular Dystrophy

